Efficiently producing green hydrogen energy on a large scale is critical to reducing the use of carbon fuel. Making this transition is tricky, though, because of the many challenges in transporting and distributing hydrogen.
A team of researchers offers a new approach: Using electrolysis to create hydrogen from wastewater and other nontraditional sources at sites close to where the hydrogen will be consumed.
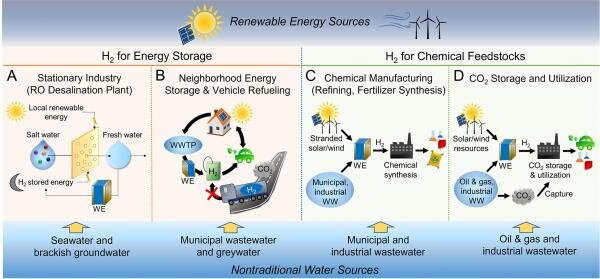
About 95% of hydrogen is made from fossil fuels. Finding good sources of water to use instead could go a long way to reducing that.
“We have all of these underutilized sources—treated wastewater, resource extraction wastewater, industrial wastewaters that we could treat,” said Lea Winter, assistant professor of chemical & environmental engineering, and lead researcher of the study. “We may not want to use them for drinking water, but we could use them for other purposes and that would save the drinking water for drinking, which is especially important for water-scarce areas.”
One way to do that is through electrolysis, a technique that uses electricity to split water into its components of oxygen and hydrogen. Most chemical processes work more efficiently at larger scales. Because the reaction in electrolysis happens only at the electrode surface, though, scale isn’t as much of a factor. That opens up the potential for efficiently using smaller and decentralized water sources, like municipal wastewater.
A more distributed infrastructure would be particularly favorable in places where water is more scarce, as it would avoid tapping into sources of drinking water. And creating hydrogen at many smaller sites instead of at a central hub means that there’s less transportation involved, one of the major costs in distributing hydrogen.
“Once you get to about 40 kilometers, then more than half of the CO2 emissions for the entire process are coming from transportation,” Winter said. “That highlights why we might want to keep this at a smaller scale. To do that, we want to be able to utilize decentralized nontraditional sources of water.”
Tapping into these sources, the researchers say, promises an abundance of energy. Extracting hydrogen from a midsize municipal wastewater treatment plant, for instance, would produce more hydrogen energy stored than a nuclear power plant. At a larger scale—a semiconductor or concrete plant, for instance—the payoff is even greater.
“The energy that we could get from extracting hydrogen from that water would be more energy than the amount consumed by U.S. vehicles on the road every year or more energy than the Three Gorges Dam,” Winter said. “If we look to different water sources and we take advantage of the water treatment technologies that we already have, then the problem of where we get the water from is really solvable for making hydrogen.”