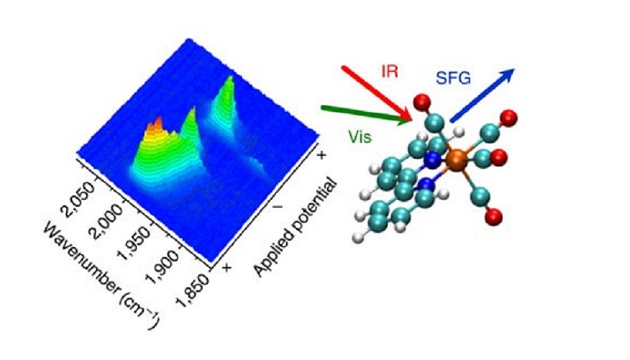
Optical spectroscopy is being applied by a team at the University of Liverpool to better understand electrocatalysis, a phenomenon that could enable more efficient conversion of waste products, like carbon dioxide (CO2), to usable energy. The team, which develops catalytic systems for sustainable fuel production, is using vibrational sum-frequency generation (VSFG) spectroscopy to study the electrochemical reduction of CO2 in situ and provide insight into the potential of electrocatalysts to convert CO2 into energy-rich byproducts such as carbon monoxide.
The researchers are using VSFG, coupled with electrochemical experiments, to explore the chemistry of one of the most promising CO2 reduction electrocatalysts, Mn(bpy)(CO)3Br. Using VSFG, the team was able to observe key intermediates that are present only at an electrode surface for a short time. This had not been possible in previous experimental studies.
Researcher Gaia Neri said, “We’ve shown that VSFG makes it possible to follow the behavior of even very short-lived species in the catalytic cycle. This is exciting as it provides researchers with new opportunities to better understand how electrocatalysts operate, which is an important next step toward commercializing the process of electrochemical CO2 conversion into clean fuel technologies.”
The team is working to further improve the sensitivity of its technique and is developing a new detection system that will allow for a better signal-to-noise ratio.