Research led by the University of Liverpool, in partnership with and Loughborough University, is making significant progress in the development of stable and practical electrolytes for lithium-oxygen batteries.
The lithium-oxygen (Li-O2) battery (or lithium-air battery), consisting of a Li-metal and a porous conductive framework as its electrode’s releases energy from the reaction of oxygen from the air and lithium. The technology is in its infancy, but in theory, could provide much greater energy storage than the conventional lithium-ion battery.
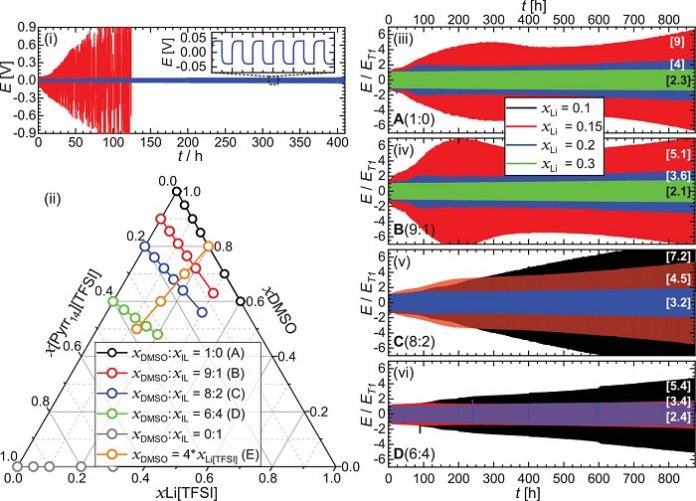
The research demonstrates that the reactivity of certain electrolyte components can be switched off by precise control of component ratios.
The ability to precisely formulate the electrolyte using readily available, low volatility components enabled us to specially tailor an electrolyte for the needs of metal-air battery technology that delivered greatly improved cycle stability and functionality.
The outcomes from our study really show that by understanding the precise coordination environment of the lithium-ion within our electrolytes, we can link this directly to achieving significant gains in electrolyte stability at the Li metal electrode interface and, consequently, enhancements in actual cell performance.
It was exciting to see through the use of both calculations and experimental data we were able to identify the key physical parameters that enabled the formulations to become stable against the lithium metal electrode interface.
The designed electrolytes provide new benchmark formulations that will support ongoing investigations within our research groups to understand and develop new, and practically viable, cathode architectures to reduce round-trip inefficiencies and further extend cycle lifetimes.
This work exemplifies a useful electrolyte design strategy for Li-air batteries underpinned by excellent science within a great collaboration. This moves us another step closer towards practical routes to overcome complex Li-air challenges.