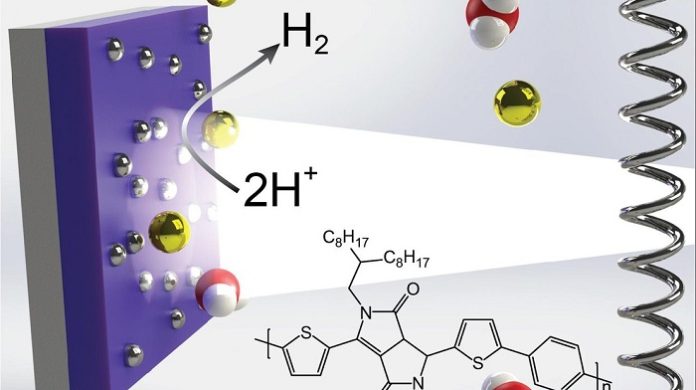
Due to their advantageous properties, organic semiconductors could be very promising photocatalysts for producing solar fuels. In fact, these materials can be synthetically tuned to absorb visible light, while simultaneously retaining energy levels that are desirable for driving various processes. While photocatalysts based on organic semiconductors have attained promising results, the understanding of the physics underpinning their functioning is still relatively limited.
Researchers at King Abdullah University of Science and Technology (KAUST), Imperial College London, and the University of Oxford has been trying to develop organic semiconductor-based photocatalysts that can efficiently harvest solar energy and could thus be used to produce hydrogen more sustainably heterojunction organic semiconductor nanoparticles can generate remarkably long-lasting reactive charges, thus they could efficiently drive sacrificial hydrogen evolution.
“We chose to use organic semiconductors to fabricate our photocatalysts because their bandgaps can be synthetically tuned to absorb strongly in the visible spectrum,” Jan Kosco, one of the researchers who carried out the study. “All else being equal, the more light a photocatalyst absorbs, the more efficiently it can convert solar energy to hydrogen.”
Most stable photocatalysts fabricated from inorganic semiconductors, such as TiO2 and SrTiO3 almost exclusively absorb UV wavelengths and have little to no activity under visible light. This can be problematic, as less than 5% of solar energy is carried via UV wavelengths. This fundamentally limits the efficiency of these inorganic semiconductor-based photocatalysts to less than 5%.
Kosco and his colleagues set out to explore the potential of organic semiconductors for driving hydrogen evolution and the photophysics underpinning their functioning further. Their study builds on their previous work on bulk heterojunction organic semiconductor nanoparticle photocatalysts.
“It is important to develop photocatalysts that are active under a broad range of UV-visible-infrared wavelengths to maximize solar light absorption,” Kosco explained. “We were initially surprised when we saw that the PM6:PCBM nanoparticles displayed higher H2 evolution rates than the PM6:Y6 NPs.”