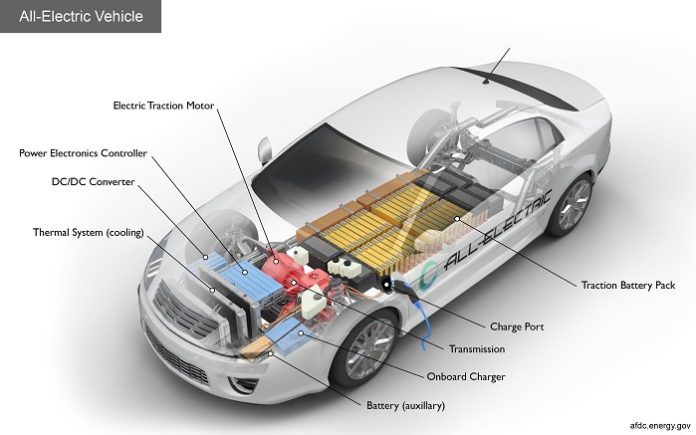
The world is moving towards electric vehicles and they will be the main driver of future mobility. Now that we all are very well aware of this fact, let us understand what the heart of an EV is really like.
A battery is a device that converts chemical energy into electric energy by means of an electrochemical reaction. An electrochemical reaction involves the transfer of electrons from one material to another in an electronic circuit.
- What types of batteries are being used in Electric Vehicles
Electric vehicles are powered by battery electric power. Different types of batteries are available in the market. They include: Lithium-Ion batteries, Solid state batteries; Nickel-Metal Hydride Batteries, Lead-Acid Batteries, and Ultracapacitors. However, Lithium-ion batteries are the most efficient and preferred. - What is a lithium-Ion battery?
Lithium-ion batteries are currently used in most electric vehicles because of their high energy per unit mass relative to other electrical energy storage systems. They also have a high power-to-weight ratio, high energy efficiency, good high-temperature performance, and low self-discharge. Most components of lithium-ion batteries can be recycled. Most of today’s Electric Vehicles use lithium-ion batteries, though the exact chemistry often varies from that of consumer electronics batteries. Research and development are ongoing to reduce their relatively high cost, extend their useful life, and address safety concerns in regard to overheating.Lithium-ion can refer to a wide array of chemistries, however, it ultimately consists of a battery based on charge and discharge reactions from a lithiated metal oxide cathode and a graphite anode. Two of the more commonly used lithium-ion chemistries are Nickel Manganese Cobalt (NMC) and Lithium Iron Phosphate (LFP). Lithium-ion batteries are used in a variety of ways, from electric vehicles to residential batteries to grid-scale applications. - What is a solid-state Battery?
The all-solid-state battery consists of a cathode composite layer, a sulphide solid electrolyte layer, and a carbon free micro-silicon anode. Before charging, discrete micro-scale Silicon particles make up the energy dense anode.They differ from lithium-ion batteries. The two differ in that a lithium-ion battery contains a liquid electrolyte while a solid-state battery—as its name suggests—features a solid one. This allows solid-state batteries to be lighter, have more energy density, offer more range, and recharge faster. - What are the other types of batteries for Electric Vehicles?
Nickel-Metal Hydride Batteries: Nickel-metal hydride batteries have a much longer life cycle than lead-acid batteries and are safe and abuse tolerant. These batteries have been widely used in Hybrid vehicles. The main challenges with nickel-metal hydride batteries are their high cost, high self-discharge and heat generation at high temperatures, and the need to control hydrogen loss.Lead-Acid Batteries: Lead-acid batteries can be designed to be high power and are inexpensive, safe, and reliable. However, low specific energy, poor cold-temperature performance, and short calendar and cycle life impede their use. Advanced high-power lead-acid batteries are being developed, but these batteries are only used in commercially available electric-drive vehicles for ancillary loads.Ultracapacitors: Ultracapacitors store energy in a polarized liquid between an electrode and an electrolyte. Energy storage capacity increases as the liquid’s surface area increases. Ultracapacitors can provide vehicles additional power during acceleration and hill climbing and help recover braking energy. They may also be useful as secondary energy-storage devices in electric-drive vehicles because they help electrochemical batteries level load power. - What is battery chemistry and how does it work?
A typical battery needs 3 parts to create electricity:• Anode – negative side of the battery.• Cathode – positive side of the battery.• Electrolyte – a chemical paste that separates the anode and cathode and transforms chemical energy into electrical energy.60% of the battery is made up of a combination of materials like zinc (anode), manganese (cathode) and potassium.Inside a battery, an electrode is a substance that is in use for the conduction of electricity. The electric current comes or leaves the non-metallic medium like an electrolytic cell. In simple language, an electrode is a conductor that helps in establishing electrical contact with a non-metallic part of the circuit. Electrodes consist of two main things that are cathode and anode which basically describe the direction of flow of current. During discharge the positive is a cathode, the negative is an anode. During charge the positive is an anode, the negative is a cathode.In a Li-ion battery the lithium ions move from the negative electrode through an electrolyte to the positive electrode during discharge and back when charging. Li-ion batteries use an intercalated lithium compound as the material at the positive electrode and typically graphite at the negative electrode. - What are the main raw materials in a battery and where do they come from?
There are five main raw materials in Li-ion batteries:•Lithium: India does not have enough lithium reserves for producing batteries and almost all Electric Vehicles in the country run on batteries imported from China. Last year 1600 tonnes of lithium reserves were found in Karnataka, apart from that, Geological Survey of India (GSI) is exploring projects in Arunachal Pradesh, Andhra Pradesh, Chhattisgarh, Jharkhand, Jammu & Kashmir and Rajasthan.•Cobalt: India is trying to acquire lithium and cobalt mines abroad. China has already taken a lead in this race. Lithium and cobalt are critical elements in batteries that power electric vehicles. The global race for these minerals is intensifying in the wake of the growing use of such vehicles. In India, occurrences of cobalt are reported from Jharkhand, Odisha, Rajasthan, Nagaland and Madhya Pradesh.•Nickel: India imports its nickel from China, UAE, US, Saudi, Switzerland, Iraq, Australia etc. In India, Odisha has the largest share of nickel ore followed by Jharkhand and Nagaland.•Manganese: India imports most of its Manganese Ore from South Africa, Australia, Gabon, Singapore, Hong Kong and Brazil. The main reserves in India are found in Karnataka, followed by Orissa, Madhya Pradesh, Maharashtra and Goa. Minor occurrences of manganese ore in Andhra Pradesh, Jharkhand, Gujarat, Rajasthan and West Bengal are also found.•Graphite: India imports its Graphite from China, Madagascar, Mozambique, Vietnam, Germany, USA, Brazil, Austria and Tanzania. In India, it’s found in Jammu and Kashmir, Gujarat, Jharkhand, Arunachal Pradesh, Karnataka, Kerala, Maharashtra, Tamil Nadu, Odisha, Chhattisgarh and Rajasthan. Some of these fields are yet to be exploited. - What impacts the range of an EV?
Much like ICE vehicles, EV range also depends on factors like speed, wind, payload, tyre traction, etc. but some battery chemistries are better than others in terms of range. Lithium-ion batteries are the most efficient ones. - Which is the most cost-effective EV battery option?
li-ion batteries are most expensive compared to Lead-Acid Batteries, Nickel-Metal Hydride Batteries and ultracapacitors but they have many advantages over the others. The advantages are: they are eco friendly, lightweight and compact, they have high energy density, low maintenance, more charge cycles, low self-discharge rate. Due to all these factors li-ion is the most widely used battery type in not just the auto industry but many other industries. The most popular electric car company Tesla also uses li-ion batteries in its vehicles.