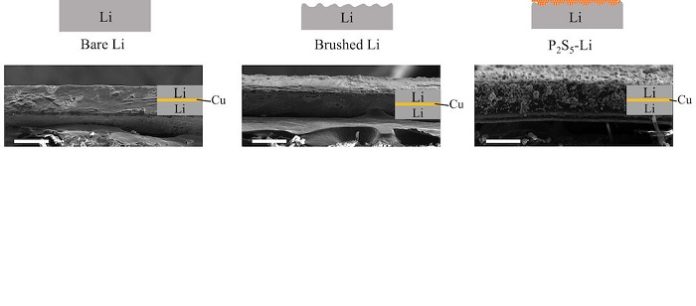
A bit of brushing may be the secret to making better rechargeable lithium batteries. The Rice University lab of chemist James Tour introduced a technique to tune the surface of anodes for batteries by simply brushing powders into them. The powder adheres to the anode and becomes a thin, lithiated coating that effectively prevents the formation of damaging dendrites.
A powder of phosphorus and sulfur ground into the surface of lithium metal foil demonstrated its surface energy can be tuned without the need for toxic solvents. Anodes so modified and paired with lithium-iron-phosphate-oxide cathodes in test cells showed they retained 70% more capacity after 340 charge-discharge cycles than off-the-shelf batteries.
“This would simplify the manufacture of high-capacity batteries while greatly improving them,” Tour said. “Sanding these powdered solids into a lithium metal anode dramatically reduces dendrite formation that can short circuit a battery, as well as the accelerated consumption of the materials.”
Lead researcher and Rice graduate student Weiyin Chen and his lab colleagues applied the necessary elbow grease to test a variety of powder candidates on their electrodes. They first brushed the surface to give it texture, then brushed in powder to create the fine film that reacts with the lithium metal and forms a solid passivation layer.
Chen and co-author Rodrigo Salvatierra, a former postdoctoral researcher and now an academic visitor in the Tour lab, constructed test batteries and determined the treated anodes retained ultralow polarization—another damaging characteristic for lithium-ion batteries—for more than 4,000 hours, about eight times longer than bare lithium anodes.
Tour said the powders effectively tune the surface energy of the electrodes, making for a more uniform behavior across the material.
“This provides a metal composite surface that prevents the loss of lithium metal from the anode, a common problem in lithium metal batteries,” Tour said. “Lithium metal batteries far exceed the capacity of traditional lithium-ion batteries, but the lithium metal is often difficult to repeatedly recharge.”
“The powder at the lithium metal surface produces an artificial passivation layer that improves the stability throughout the charge-discharge cycles,” Chen said. “Using this brush-on method, the metal surface is stabilized so that it can be safely recharged.”
To show the technique may have wider application, the lab also ground powder into a sodium electrode and discovered the process greatly stabilized its voltage overpotential.
The research aligns with the recent discovery by Tour and Rice mechanical engineer C. Fred Higgs III that sanding certain powders into surfaces can make them superhydrophobic, or highly resistant to water.