Gaining sustainable energy from wind, solar, and water is commonly known and applied. However, renewable sources depend on environmental conditions: in peak times of wind and sun, excess energy is produced that is needed in times of less wind and sunshine. But how do store and transport this excess energy efficiently?
So far, no reliable, safe, and cheap way has been found to store a high amount of energy in a small volume container. Now, scientists from the Max-Planck-Institut für Eisenforschung (MPIE) and the Eindhoven University of Technology analyzed how metals, particularly iron, can be used for energy storage and which parameters determine the efficiency of the storage and reuse.
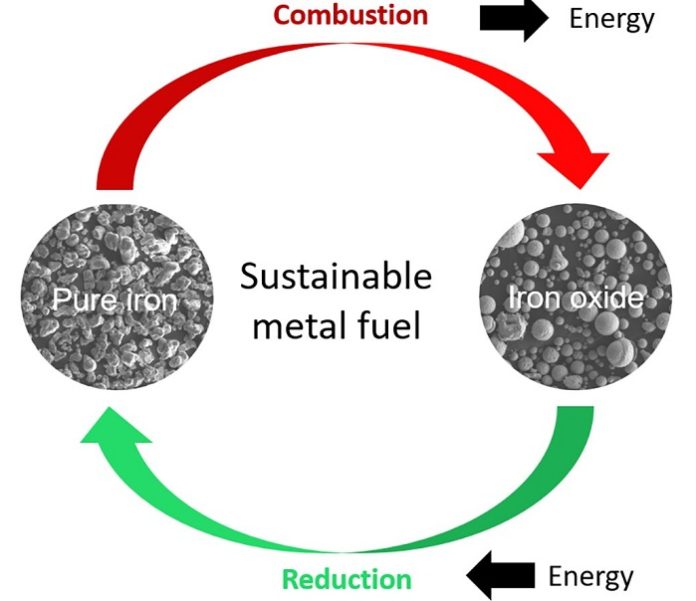
Creating a circular reduction and combustion process
“Storing energy in metals and burning them to free the energy whenever needed is a method already applied in aerospace technology. Our aim was to understand what exactly happens at the micro- and nanoscale during the reduction and combustion of iron and how the microstructure evolution influences the efficiency of the process. Additionally, we wanted to find how to make this process circular without losses in energy or material,” explains Dr. Laurine Choisez, who recently finished her postdoctoral research at the MPIE.
When iron ores are reduced to iron, a lot of energy is naturally stored in the reduced iron. The idea is to get this energy out of the iron whenever needed by oxidizing the iron back to iron oxide. In times of excess energy from wind, sun, or water, this iron ore could be again reduced to iron and the energy stored.
The scientists speak of combustion when describing the “burning,” meaning oxidation, of the iron back to iron ore. Choisez and her colleagues at MPIE focused on the characterization of the iron powders after reduction and combustion using advanced microscopy and simulation methods to analyze the powder purity, morphology, porosity, and thermodynamics of the combustion process.
The obtained microstructure of the combusted iron powders is decisive for the efficiency of the following reduction process, and to determine whether the process of reduction and combustion is fully circular, meaning that no additional energy or material has to be added.
The scientists present two combustion pathways, one supported by a propane pilot flame and one self-sustained in which the only fuel used is the iron powder, and show how the combustion pathway influences the microstructure of the combusted iron.
“We are currently upscaling the reduction and combustion steps to an industrial relevant level determining the exact parameters like temperature and particle size, which are needed,” explains Niek E. van Rooij, doctoral researcher in the Combustion Technology group of the Eindhoven University of Technology.
A recent study showed that using metals to store energy is feasible. Future studies will now analyze how to increase the circularity of the process, as the size of some combusted particles is decreased compared to their original size due to partial iron evaporation, micro-explosions, and/or fracture of some iron oxide particles.