The cost of harvesting solar energy has dropped so much in recent years that it’s giving traditional energy sources a run for their money. However, the challenges of energy storage—which require the capacity to bank an intermittent and seasonally variable supply of solar energy—have kept the technology from being economically competitive.
Cornell University researchers have been exploring the use of low-cost materials to create rechargeable batteries that will make energy storage more affordable. Now, they have shown that a new technique incorporating aluminum results in rechargeable batteries that offer up to 10,000 error-free cycles.
This new kind of battery could provide a safer and more environmentally friendly alternative to lithium-ion batteries, which currently dominate the market but are slow to charge and have a knack for catching fire.
Among the advantages of aluminum is that it is abundant in the earth’s crust, it is trivalent and light, and it, therefore, has a high capacity to store more energy than many other metals. However, aluminum can be tricky to integrate into a battery’s electrodes. It reacts chemically with the glass fiber separator, which physically divides the anode and the cathode, causing the battery to short circuit and fail.
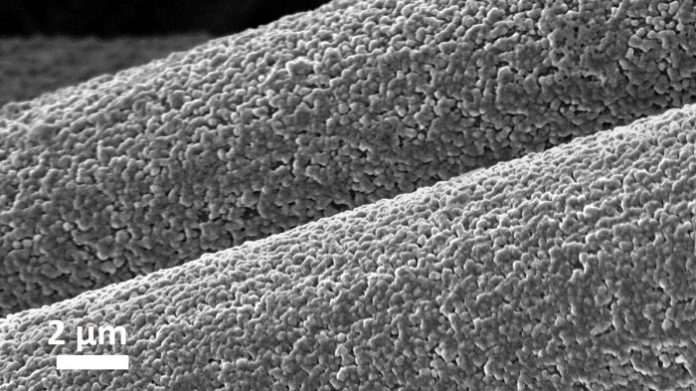
The researchers’ solution was to design a substrate of interwoven carbon fibers that form an even stronger chemical bond with aluminum. When the battery is charged, the aluminum is deposited into the carbon structure via covalent bonding, i.e., the sharing of electron pairs between aluminum and carbon atoms.
While electrodes in conventional rechargeable batteries are only two-dimensional, this technique uses a three-dimensional—or nonplanar—architecture and creates a deeper, more consistent layering of aluminum that can be finely controlled.
The aluminum-anode batteries can be reversibly charged and discharged one or more orders of magnitude more times than other aluminum rechargeable batteries under practical conditions.